Banshan™: Engineered for Rigorous Sterile Environments
Banshan™ Material is an innovative high-tech enterprise specializing in the research, development, production, and supply of advanced materials and packaging solutions, particularly for sterile medical applications and high-performance industrial uses. The company focuses on high-density polyethylene (HDPE) and flash-spun specialty materials that deliver excellent protection, sterility compatibility, and sustainability across healthcare, pharmaceutical, protective equipment, and other industrial sectors. Their products are engineered to support sterilization processes, product safety, regulatory compliance, and eco-friendly performance across global supply chains.
In modern healthcare manufacturing, packaging is not a secondary consideration it is a critical component of product safety, regulatory compliance, and clinical performance. Medical devices must reach hospitals and laboratories in a sterile, undamaged, and compliant condition. This is only possible through engineered medical device packaging solutions that protect against contamination, physical damage, moisture, and environmental stress throughout the supply chain.
Solutions offered under Banshan™ Materials sterile medical protection applications are designed specifically for healthcare environments where sterility, durability, and regulatory integrity are non-negotiable.
This guide outlines the importance, structure, and applications of:
- Medical device packaging solutions
- Sterile packaging systems
- Surgical packaging
- Medical grade packaging
- Medical packaging products
- Medicinal grade packaging bags
1. Medical Device Packaging Solutions
Medical device packaging solutions are specialized packaging systems developed to protect devices during:
- Sterilization processes
- Storage and warehousing
- Global transportation
- Clinical handling and use
These solutions function as both protective barriers and sterile barrier systems (SBS), ensuring that products such as implants, surgical tools, diagnostic kits, and consumables remain contamination-free until point of use.
A complete packaging solution typically includes:
- Primary sterile barrier packaging
- Secondary protective packaging
- Validation for sterilization compatibility
- Seal integrity assurance
- Regulatory documentation support
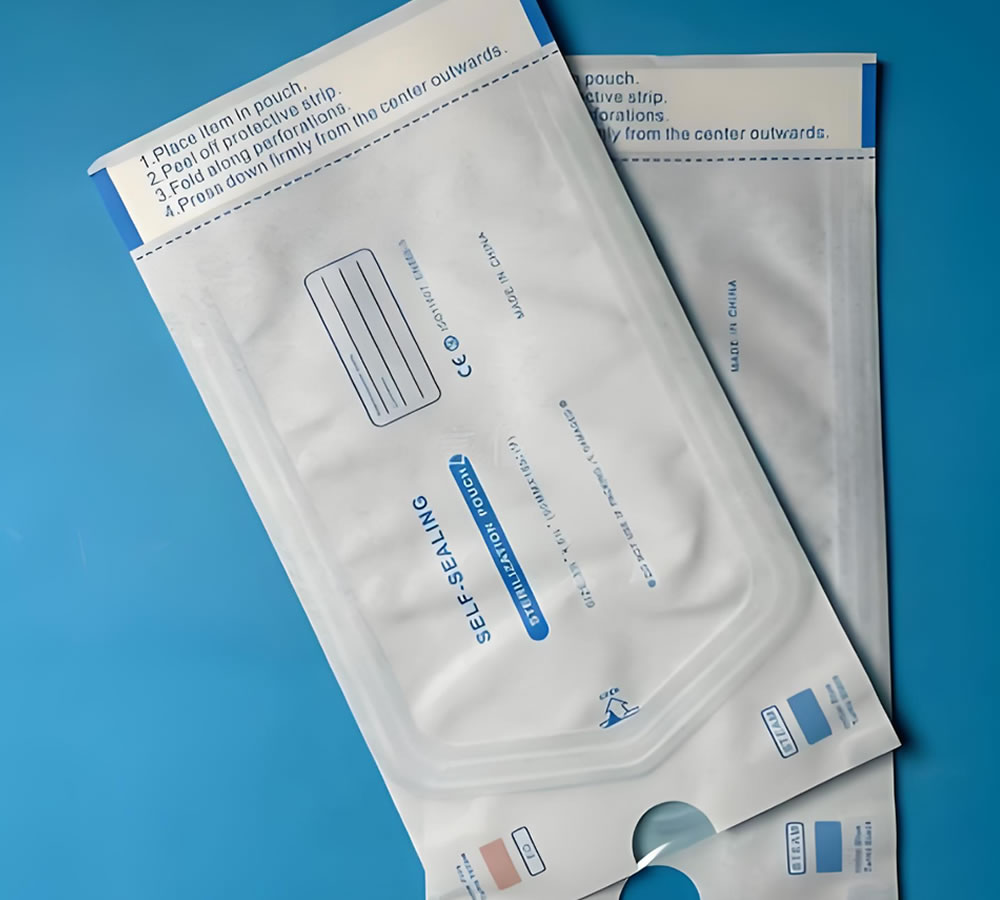
2. Sterile Packaging Systems
Sterile packaging is engineered to prevent microbial ingress after sterilization. Once a product is sterilized, the packaging must maintain sterility until the moment it is opened in a clinical environment.
Key Functions
- Acts as a microbial barrier
- Maintains sterility during distribution
- Withstands sterilization methods
- Provides aseptic presentation
Critical Features
- Controlled seal strength
- Breathable yet protective materials
- Puncture and tear resistance
- Sterilization indicator compatibility
Sterile packaging is fundamental in preventing hospital-acquired infections and ensuring device performance reliability.
3. Surgical Packaging
Surgical packaging solutions are tailored for devices used in operating rooms and procedural environments where sterility and handling efficiency are essential.
Applications include:
- Surgical instruments
- Disposable procedure kits
- Implants and sutures
- Sterile gowns and drapes
Design Requirements:
- Easy aseptic opening
- Clear content visibility
- Reliable peel performance
- High seal integrity
Surgical packaging must balance sterile protection with usability, allowing clinical staff to access devices quickly without compromising sterile fields.
 4. Medical Grade Packaging
4. Medical Grade Packaging
Medical grade packaging refers to materials and packaging systems manufactured under strict quality and cleanliness standards to meet healthcare regulations.
Material Characteristics
- Biocompatible
- Low particulate generation
- Chemical and moisture resistance
- Compatible with sterilization
Such compliance ensures packaging maintains its sterile barrier function throughout the product lifecycle.
5. Medical Packaging Products
Medical packaging products encompass a broad range of sterile and protective formats:
| Product Type | Primary Function |
| Sterilization Pouches | Instrument sterilization and storage |
| Medical Grade Films | Moisture and contamination protection |
| Cleanroom Bags | Sensitive device protection |
| Surgical Kit Packaging | Procedure-specific sterile kits |
These products are often customized based on device geometry, sterilization method, and transportation requirements.
6. Medicinal Grade Packaging Bags
Medicinal grade packaging bags are high-barrier bags used to protect medical and pharmaceutical products requiring superior contamination control.
Performance Benefits
- Excellent oxygen and moisture barrier
- Long-term storage stability
- Sterilization compatibility
- High seal strength
These bags are commonly used for:
- Pre-sterilized surgical kits
- Diagnostic device components
- Pharmaceutical support products
They are essential for maintaining product integrity in global healthcare distribution networks.
7. Importance of Packaging Validation
Medical packaging must undergo extensive validation to ensure performance under real-world conditions.
Validation activities include:
- Seal strength testing
- Burst and creep testing
- Microbial barrier testing
- Transportation simulation
- Aging studies
These processes verify that packaging systems maintain sterility and structural integrity throughout shelf life.
8. Benefits of Professional Medical Packaging Solutions
- Maintains device sterility
- Enhances patient safety
- Reduces contamination risk
- Improves handling efficiency
- Supports regulatory compliance
- Protects brand reputation
9. Industry Challenges
Medical packaging providers must address:
- Evolving regulatory requirements
- Sustainability demands
- Complex device geometries
- Global distribution stress factors
Advanced material science and engineered packaging designs help overcome these challenges.
What are medical device packaging solutions?
Medical device packaging solutions are specialized packaging systems designed to protect medical devices from contamination, physical damage, moisture, and environmental exposure while maintaining sterility throughout storage, transportation, and clinical use.
Why is sterile packaging important in healthcare?
Sterile packaging prevents microbial contamination and ensures that medical devices remain sterile until the point of use, reducing the risk of infections and ensuring patient safety.
What is the difference between sterile packaging and surgical packaging?
Sterile packaging focuses on maintaining microbial barrier integrity after sterilization, while surgical packaging is specifically designed for devices used in operating rooms, emphasizing aseptic presentation, easy opening, and handling efficiency.
What materials are used in medical grade packaging?
Medical grade packaging typically uses materials such as medical-grade paper, high-density polyethylene (HDPE), multilayer films, and specialty laminates that are compatible with sterilization and meet regulatory standards.
What are medical packaging products?
Medical packaging products include sterilization pouches, Tyvek rolls, medical-grade bags, films, cleanroom packaging, and customized sterile barrier systems designed for different device types and sterilization methods.
What is a medicinal grade packaging bag used for?
Medicinal grade packaging bags are high-barrier bags used to store and transport medical and pharmaceutical products that require superior protection against moisture, oxygen, and contamination.
Which sterilization methods must medical packaging withstand?
Medical packaging systems are typically validated for Ethylene Oxide (EO), Gamma radiation, Electron Beam (E-beam), and in some cases steam sterilization, depending on material compatibility.
What standards regulate medical device packaging?
Medical packaging must comply with international standards such as ISO 11607 and EN 868, which define requirements for sterile barrier systems and packaging validation.
How does packaging impact medical device safety?
Compromised packaging can lead to contamination, device damage, or sterility loss, directly affecting clinical performance and patient safety.
Can medical packaging be customized?
Yes, medical packaging solutions can be customized based on device size, shape, sterilization method, and regulatory requirements to ensure optimal protection and performance.